Revolutionizing Medicine: The Future of RNA Therapeutics
Written on
Chapter 1: A New Era in Medicine
Throughout history, medicine has occasionally undergone transformative changes, often unnoticed at the moment they occur. While I wasn't present for Alexander Fleming's discovery of penicillin in 1928 or for the revelation of DNA's double-helix structure by Watson, Crick, and Franklin in 1953, I can identify two pivotal advancements in contemporary medicine that will likely be recognized as major milestones in retrospect. One is the rise of artificial intelligence, which has garnered considerable excitement and speculation about its potential impact. The other, which may be less visible yet equally significant, is RNA therapeutics—the key to future medications.
To understand this shift, consider that many diseases stem from protein-related issues. For instance, hypercholesterolemia results from excessive protein production, while hemophilia arises from insufficient protein levels. Viewing diseases through this lens reveals that current treatments typically intervene late in the disease process. Conventional medications often aim to inhibit protein-receptor interactions, block protein cleavage, or accelerate protein breakdown, all of which are reactions that occur after the problem—namely, the production of harmful proteins—has already begun.
This is where small interfering RNAs (siRNAs) come into play. First identified in 1998 by Andrew Fire and Craig Mello at UMass Worcester, siRNAs earned the Nobel Prize in Medicine just eight years later, underscoring their significance. In contrast, Kariko and Weissman, who developed mRNA vaccines 18 years prior, received the Nobel Prize only recently.
siRNAs represent our body's mechanism for eliminating proteins before they are produced. These RNA molecules are approximately 20 base pairs long and target complementary mRNA strands, binding to them and recruiting proteins to facilitate their destruction. As a result, the targeted mRNA is degraded, preventing protein synthesis altogether.
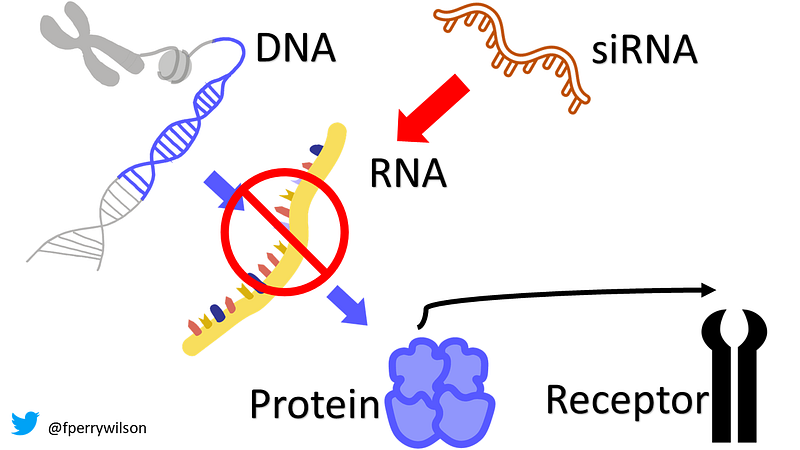
This leads to a crucial question: How do we harness siRNAs effectively? A recent study published in JAMA illustrates a compelling application.

Lipoprotein A (Lp(a)), a protein similar to LDL, is linked to cardiovascular diseases, including heart attacks and strokes. Unfortunately, traditional treatments like statins and dietary changes have minimal impact on Lp(a) levels, which appear to be determined by genetics.
What if we could halt the genetic machinery responsible for Lp(a) production? Enter lepodisiran, a groundbreaking drug from Eli Lilly. Unlike many medications derived from natural sources, lepodisiran was designed from the ground up. Thanks to the Human Genome Project, we now have the genetic blueprint for Lp(a), allowing for the straightforward creation of a specific siRNA to target it. This simplicity is a hallmark of siRNA technology: instead of searching for compounds that bind to proteins with potential side effects, we can simply inhibit a desired protein's synthesis.
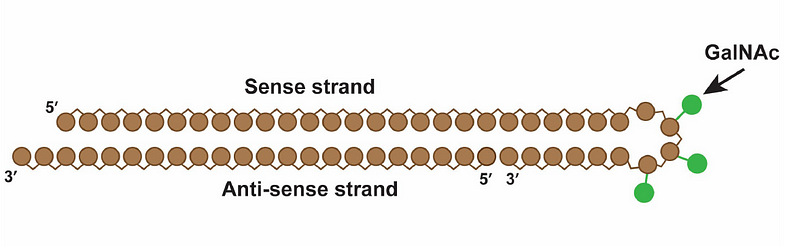
However, it's important to note that siRNAs degrade rapidly in the body, necessitating careful targeting to the liver, where Lp(a) is synthesized. Lepodisiran includes a specialized targeting label to direct it to liver cells.
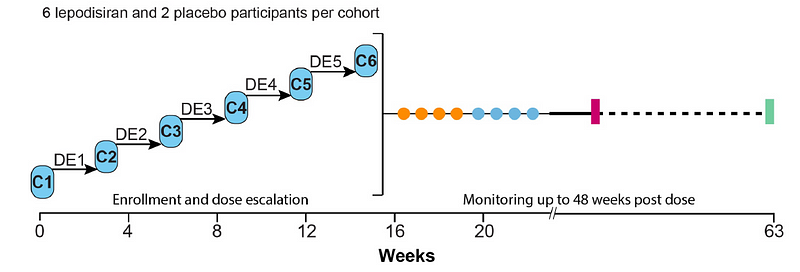
The study employed a dose escalation protocol with six participants who had elevated Lp(a) levels. They began with a 4mg dose (two additional subjects received a placebo) and were closely monitored over several days, with subsequent dose increases for additional participant groups. This process continued until a maximum dose of 608mg was reached.
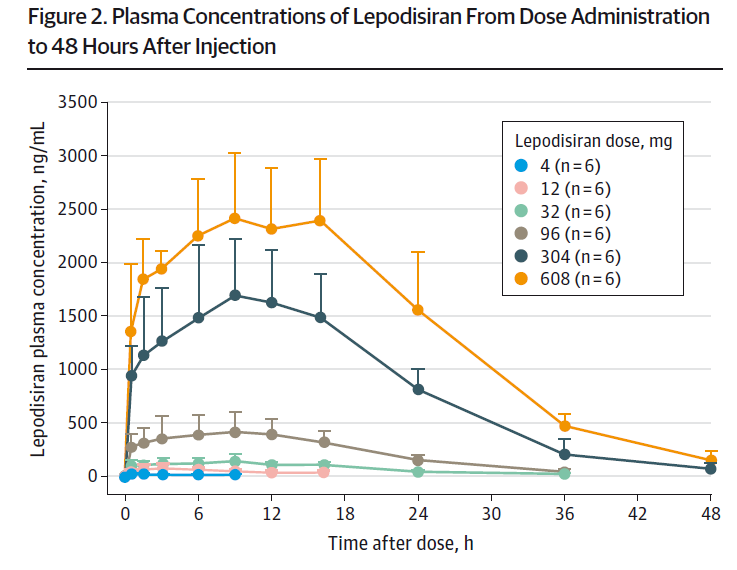
Lepodisiran requires an injection, as siRNA cannot survive the digestive system. Remarkably, within about 48 hours, the drug was undetectable in the bloodstream following a single injection.
The results are astounding. Following just one injection, Lp(a) levels began to decline within a week and remained significantly reduced. In higher-dose cohorts, levels were nearly undetectable a year later.
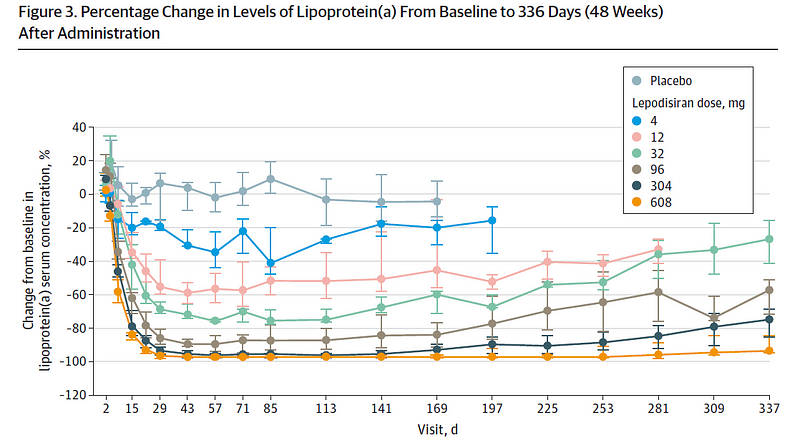
This single injection's capacity to suppress protein synthesis for an entire year is revolutionary. If validated, this could redefine treatment paradigms.
It is worth mentioning that the study's primary focus was safety, not outcomes like heart attacks or strokes; however, the drug was generally well tolerated, with no significant difference in adverse events between treatment and placebo groups. A genuine concern might be whether it’s safe to reduce Lp(a) levels to zero for an extended period. While lower doses exhibited less pronounced effects, the implications of these drugs are profound.
The landscape of medicine is shifting. For instance, a recent study published in the New England Journal of Medicine examined Zilebesaran, another siRNA that inhibits angiotensinogen to manage blood pressure. As with lepodisiran, a single injection resulted in substantial angiotensinogen suppression and sustained blood pressure reduction.
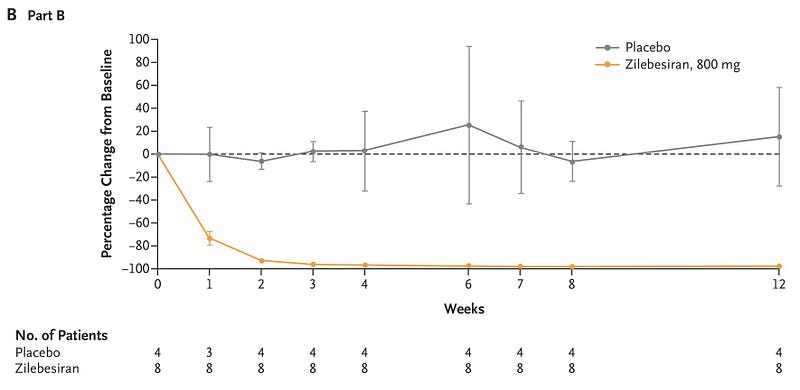
Imagine a future where an annual visit to the doctor could involve receiving RNA therapies, eliminating the need for multiple medications. This reality may not be far off—perhaps just five years away.
It seems clear that the quick recognition of siRNA technology with a Nobel Prize was well warranted.
A version of this commentary first appeared on Medscape.com.
Chapter 2: The Future of Personalized Medicine
The first video illustrates how RNA-based medicines will revolutionize future treatments, emphasizing the innovative nature of RNA therapeutics.
The second video discusses RNA therapeutics and their potential to personalize medicine, offering a glimpse into future healthcare possibilities.