Understanding Neanderthals' Role in Covid-19 Genetics
Written on

For the past six months, geneticists worldwide have been investigating whether variations in human genomes might influence the risk or severity of Covid-19 infections. To date, researchers have identified a specific segment on chromosome 3 as a potential factor. Individuals in Italy and Spain who experienced severe respiratory issues due to Covid-19 possess a particular "risk" allele in the 3p21.31 region approximately 1.7 times more frequently than the general population.
Recent findings link this risk allele to our Neanderthal ancestors.
As a researcher in human evolution, I find it thrilling when such discoveries have contemporary significance. However, in the context of Covid-19, sensationalism can be misleading. Some individuals might mistakenly believe their Neanderthal DNA from ancestry tests could indicate their Covid-19 risk, which is not accurate.
Environmental factors and personal behaviors play a far more substantial role in the virus's transmission than genetics. Currently, the most effective methods to curb the virus's spread involve social distancing and wearing masks. Ultimately, vaccine development offers the best long-term strategy for overcoming Covid-19.
While human genetics may provide insights into the virus's biology and its threat level, understanding how genes influence infection can clarify why many remain asymptomatic or why certain populations are less affected.
The connection to Neanderthal DNA adds a historical perspective. Covid-19 is a new pathogen interacting with human biology that has evolved over the past half million years.
Identifying relevant human genes for Covid-19 susceptibility is challenging. Most people associate genes with straightforward traits like blood types, a concept largely taught in high school biology, which emphasizes a single genotype determining a trait.
However, only a few human traits follow such simple inheritance patterns. For the majority, hundreds or even thousands of genes contribute to variation, with each gene exerting a minor influence, making them difficult to detect.
Human geneticists utilize genome-wide association studies (GWAS) to pinpoint small gene-trait associations. These studies analyze millions of genotypes across thousands of individuals, with larger sample sizes allowing for the identification of smaller genetic effects.
Currently, leading GWAS studies involve large cohorts, such as the UK Biobank, which has compiled DNA and health records from 500,000 participants. For traits measurable across this population, researchers have identified thousands of genes with minimal impacts. The vast size of these studies enables researchers to account for other influencing factors like age, socioeconomic status, or geographic location.
Studying emerging diseases with GWAS is difficult due to the challenge of assembling large sample sizes. The Covid-19 Host Genetics Initiative is monitoring genetics research on Covid-19 cases globally. As of the latest Covid-19 hg release (June 2020), the UK Biobank contained only 1,190 confirmed Covid-19 patients. Thus, the available genetic data from Covid-19 patients is still too limited to uncover many relevant human genes related to infection or disease progression.
An early genetic analysis published in the New England Journal of Medicine examined about 1,500 severe Covid-19 patients in Italy and Spain, led by David Ellinghaus. This study found two genomic regions linked to heightened Covid-19 risk, with one region—the ABO gene locus, responsible for blood types—drawing significant attention. The findings suggested that type A blood might correlate with up to a 50% greater risk of severe Covid-19 symptoms.
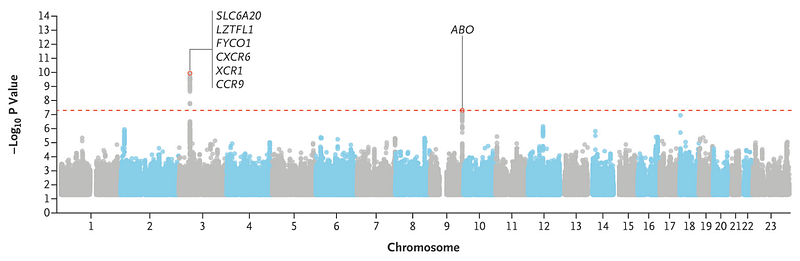
However, this ABO finding has become a cautionary example of hasty conclusions based on small sample sizes. The Covid-19 hg site has since updated to include studies involving 6,000 Covid-19 patients across Europe, indicating that ABO blood type does not significantly influence risk in this larger group.
For over a century, scientists have explored blood types, and numerous flawed studies have associated ABO with unrelated traits. Although the link to Covid-19 initially seemed plausible, it now appears inaccurate. Future research may clarify whether ABO does impact Covid-19 in specific contexts, but for now, this case highlights that gene association claims often turn out to be incorrect.
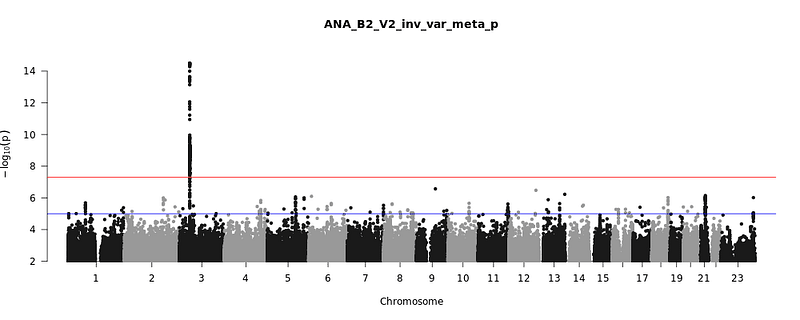
The Ellinghaus study did identify a genetic risk factor that remains consistent. The correlation between the 3p21.31 region and severe Covid-19 was notably strong for a polymorphism known as rs11385942. Individuals with an additional adenine at this site were approximately 1.77 times more likely to fall into the severe Covid-19 category.
While this figure may seem substantial, it actually reflects a minor portion of the variance in Covid-19 risk within the population. In the examined European groups, this extra "A" allele occurs in about 8% of individuals, with around 15% carrying at least one copy, compared to nearly 24% among the Italian and Spanish Covid-19 patients. Nonetheless, 76% of severe Covid-19 patients in these samples do not possess this "risk" allele.
It's essential to recognize that early GWAS studies may overlook critical factors. Researchers analyzing samples of severe Covid-19 cases struggle to adjust for variables like socioeconomic status, age, or other influences that could affect how host genes interact with the virus.
Even if this gene discovery offers limited predictive power concerning Covid-19 risk, it can serve as a foundation for further investigations into the disease's biology. To delve deeper, geneticists must scrutinize the 3p21.31 region more thoroughly. GWAS alone does not pinpoint which gene variant directly influences a trait or how it operates. The positive GWAS result at 3p21.31 indicates researchers are on the right track, but even the most significant finding, the extra "A," may simply be a coincidental marker linked to a nearby genetic change impacting Covid-19.
This region encompasses multiple genes of interest. The rs11385942 variant is situated within an intron of the LZTFL1 gene. Nearby, CCR9 and CXCR6 produce chemokines, which are signaling molecules that attract immune cells to infection sites. A well-known chemokine gene variant, CCR5, offers strong resistance to HIV. It's possible that similar immune mechanisms underlie the association between 3p21.31 and Covid-19 or may reveal different aspects of host response to the virus.
Further research is necessary to determine whether the heightened risk stems from increased susceptibility to initial infection, greater likelihood of symptomatic progression, or accelerated onset of respiratory failure—potentially all three.
Recent advancements in human genomics reveal that as researchers study larger patient samples, they will identify additional genomic regions relevant to Covid-19, with increasingly subtle effects. The collective impact of numerous genes may provide a more comprehensive understanding than focusing solely on the single locus currently under scrutiny.
So where do Neanderthals fit into this narrative?
The authors of the recent study, Hugo Zeberg from the Karolinska Institutet and Svante Pääbo of the Max Planck Institute for Evolutionary Anthropology, examined the 3p21.31 region to identify which SNP alleles are present in ancient Neanderthal genomes. Over the past decade, Pääbo's team has sequenced high-quality genomes from three Neanderthals: Vindija 33.19 from Croatia, the Altai Neanderthal from Denisova Cave in Russia, and Chagyrskaya 8 from Chagyrskaya Cave in Russia. All these Neanderthal genomes share the extra "A" allele linked to Covid-19 risk in the Ellinghaus study.
Particularly, the Vindija 33.19 individual possessed 12 out of the 14 associated SNP alleles for Covid-19 risk in the 3p21.31 region. This strong correlation suggests that the haplotype containing these SNP alleles may have originated with Neanderthals. The Altai and Chagyrskaya specimens have fewer of these alleles but appear to possess closer relatives of this risk haplotype than most contemporary individuals lacking it. However, the Denisovan genome did not show the SNP alleles associated with Covid-19 risk.
Simultaneously, Zeberg and Pääbo reviewed a global genome sample database. Their preprint reports that the Neanderthal risk haplotype is rare or absent in most East and Southeast Asian populations and completely absent in sub-Saharan Africa. In Europe, the haplotype occurs at a modest frequency of about 5–10% and is similarly low in Native American samples. In present-day samples from India, Pakistan, and Bangladesh, however, this haplotype is quite prevalent, reaching over 40% in some areas.
However, this aspect of their analysis raises questions. Zeberg and Pääbo assert that the "Neanderthal core haplotype" does not exist in sub-Saharan Africa or East Asia. Yet, data from the 1000 Genomes Project indicate that the extra "A" allele is present in African population samples at around 5%. It's possible that some African individuals inherited this allele independently of the Neanderthal haplotype shared by many modern individuals. Alternatively, the Neanderthal haplotype may exist in a significant number of Africans that the preprint did not account for, casting doubt on the relevance of the Neanderthal connection to Covid-19 risk. Hopefully, Zeberg and Pääbo will clarify this discrepancy before submitting their research for publication.
It's vital to understand that inheriting a gene from Neanderthals isn't extraordinary. Evolution capitalizes on any genetic variation that confers an advantage in a given environment. With approximately 2% of modern human genomes tracing back to Neanderthals, it's reasonable to anticipate that one in fifty GWAS findings will likely involve Neanderthal gene variants.
Neanderthal genes reflect half a million years of adaptation to their unique environments, which differed from those of contemporary Africans in several respects, such as seasonality, temperature, sunlight exposure, and available food sources. Their social and disease environments might also have varied in ways distinct from those in Africa, although significant overlap exists.
Stephen Jay Gould once proposed that if we could rewind evolutionary history and restart it, the outcome would differ significantly. His view suggests that evolution exploits random variations, implying that chance and contingency dominate the long-term process. Our Neanderthal and African ancestors diverged from a shared gene pool over 500,000 years ago, leading each population down its unique evolutionary path.
Certain genetic changes in Neanderthals might have been ineffective or harmful had they occurred in Africans. While these adaptations were beneficial or tolerated in the Neanderthal environment, they may not have been advantageous in Africa. In such instances, we could argue that restarting evolution would yield no difference. Conversely, some genetic changes are notably prevalent in modern humans. The so-called "Neanderthal introgression deserts" are regions of human chromosomes devoid of sequences resembling those of Neanderthals.
In contrast, specific areas of the genome exhibit an unexpected abundance of Neanderthal sequences among contemporary humans. One such example is the haplotype across 3p21.31, which has been found in over 40% of some South Asian populations. These prevalent Neanderthal variations suggest scenarios where restarting evolution could have led to different outcomes. Africans might have evolved this gene, but the Neanderthals did instead. This occurrence could reflect a specific advantage relevant to the Neanderthal environment or simply be a matter of chance.
What we have gleaned from Neanderthal DNA underscores an evolutionary strategy that Gould did not consider: the creation of a "mix tape." Through repeated intermingling and hybridization among ancient branches of our family tree, one population could acquire favorable mutations from another. Modern humans assimilated some of the beneficial traits from our relatives' evolution.
Over the past decade since the first Neanderthal genome was sequenced, we have learned much about the significance of their genes for our recent ancestors. Many prevalent Neanderthal genes today relate to immunity or pathogen defense. This includes variations within the human leukocyte antigen (HLA) gene system, a Neanderthal haplotype within the OAS1, OAS2, and OAS3 genes that encode enzymes crucial for innate immunity against RNA viruses, as well as a variant of the STAT2 gene connected to innate immunity.
In 2018, David Enard and Dmitri Petrov analyzed over 4,000 proteins known to interact with viruses, discovering that the genes encoding these proteins were more likely to include Neanderthal introgressed sequences compared to other genomic regions. While their study did not specifically address coronaviruses, these also fall under the category of RNA viruses.
Such findings offer fascinating insights into our biological and historical narratives. Researchers are beginning to revise our understanding of viruses and their evolutionary significance. Viruses often evade the attention of paleontologists focused on ancient skeletal remains. Currently, the most exciting developments in Neanderthal research include in vitro analysis of cells expressing Neanderthal genes, highlighting the evolution of human scientific inquiry.
This progress underscores the importance of avoiding outdated genetic concepts in the context of a new pandemic. Epidemiologists consistently stress that the most effective strategies for reducing Covid-19 transmission involve social distancing and mask-wearing to limit aerosol spread. No genetic factor guarantees immunity.
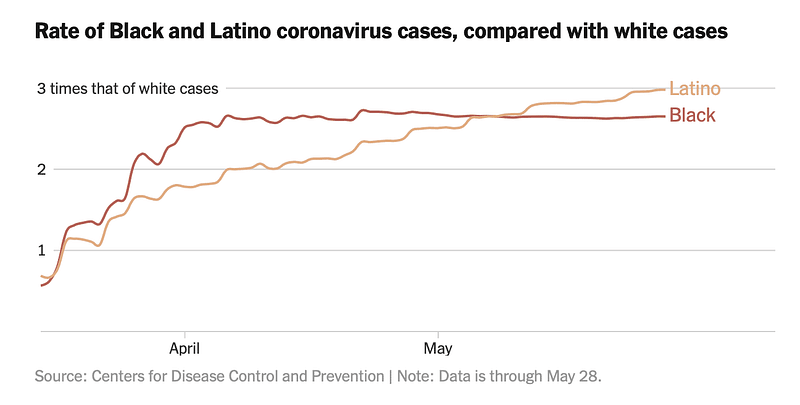
Currently, the virus continues to spread rapidly in the United States, and individuals’ Neanderthal ancestry does not appear to significantly affect infection rates. The highest incidence is observed among Black and Latino populations, largely attributable to increased transmission in urban settings and, more recently, in states like Texas, Florida, and Arizona. The Navajo Nation and several other tribes have also faced high infection rates. Epidemiologists identify numerous factors contributing to the disparity in Covid-19 transmission among these groups, including multigenerational households, reliance on public transportation, obesity, long-term health issues, and a higher percentage of individuals working in frontline occupations.
None of these influences are genetic. Behavioral and cultural dynamics primarily drive this pandemic, many of which are compounded by a legacy of poverty and systemic racism.
While further investigation into host genetics related to Covid-19 may provide insights into some of the virus's enigmas—such as why many infected individuals remain asymptomatic or why certain individuals become superspreaders—it's crucial to remain clear about the current pandemic reality. No genetic test can accurately predict Covid-19 risk. General ancestral lineage does not confer immunity to infection. Although Neanderthals are an intriguing topic for research, they are not a determinant of individual health outcomes.