Understanding the Plastic Dilemma: Insights from the Molecular Viewpoint
Written on
Chapter 1: The Ubiquity of Plastics
Imagine a world devoid of plastic. Picture your daily life without the plastic keyboard beneath your fingers, the chair you sit on, or even the frames of your glasses. The reality of modern living is that plastics have infiltrated every aspect of our existence. Our technological advancements rely heavily on these materials, making life in the 21st century almost unimaginable without them.
The journey of plastics began in 1907 with the creation of bakelite, a phenol-formaldehyde resin that was not only durable but also an excellent insulator. This groundbreaking invention marked the dawn of the 'Polymer Age', paving the way for the wide array of plastics we use today. By the late 20th century, plastics had become ubiquitous, celebrated for their durability, flexibility, and affordability. However, this very durability has led to an unforeseen dilemma: their longevity in our environment.
Chapter 2: The Molecular Makeup of Plastics
Plastics are composed of long chains of molecules known as polymers. While we are adept at synthesizing a wide variety of these polymers, they also exist in nature in forms such as proteins and DNA.
Polymers are formed from smaller units called monomers, which bond chemically to create these extensive chains. The nature of the monomers involved and their interactions determine the material's physical properties. To enhance rigidity, chemical cross-links can be introduced, as seen in the rubber of elastic bands, preventing the chains from moving freely.
Section 2.1: The Challenge of Plastic Degradation
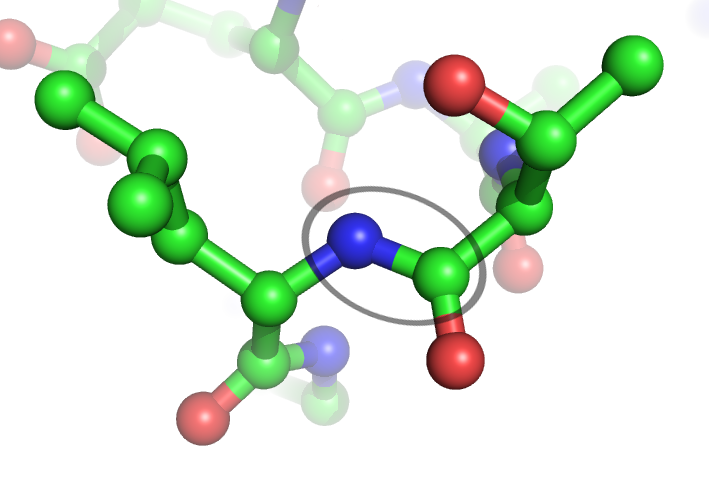
Some polymers can decompose relatively easily, as seen when leaves break down in autumn. This decomposition occurs due to the relatively reactive bonds between the monomers. For instance, enzymes known as proteases can effectively break down polypeptide chains in proteins.
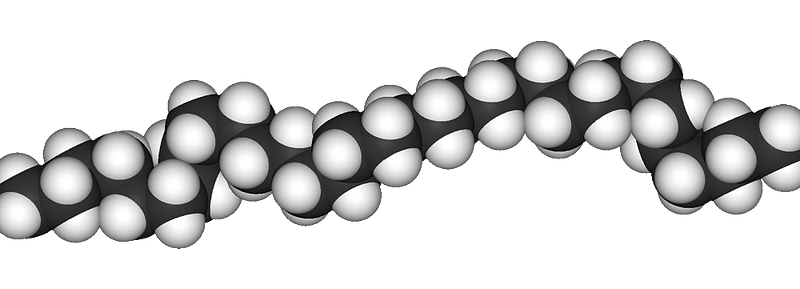
In contrast, many commonly used plastics are addition polymers, characterized by strong, unreactive carbon-to-carbon bonds. Examples include polyethylene, PVC, and polystyrene, which resist natural degradation.
Section 2.2: The Environmental Impact of Plastic Longevity
While the durability of plastics can be seen as advantageous, it poses significant environmental challenges. According to National Geographic, plastics can take up to 400 years to decompose fully. The repercussions are evident worldwide, as plastic waste threatens marine and avian life and disrupts various ecosystems. Although larger plastic debris can be collected, microplastics—tiny fragments resulting from environmental factors—are pervasive and difficult to remove, even affecting the reproductive systems of some species.

Chapter 3: The Production and Disposal of Plastics
The conventional manufacturing of plastics presents additional environmental issues. Plastics like polyethylene are derived from ethylene, which originates from the petrochemical industry reliant on fossil fuels. Thus, the plastic problem is twofold: the reliance on fossil fuels for production and the difficulties in environmentally safe disposal.
Section 3.1: Bioplastics as a Potential Solution
Bioplastics, created from plant and algal sugars, offer an alternative that bypasses fossil fuel dependency. However, they remain relatively costly to produce and sometimes require extensive land use. Currently, bioplastics do not match the strength of their oil-based counterparts.

From a chemical standpoint, bioplastics are more susceptible to degradation due to their more reactive bonds. Yet, they often need specific conditions, such as ample oxygen, to break down properly. If disposed of in landfills, bioplastics can take decades to decompose and may release methane, a potent greenhouse gas.
Reducing plastic consumption, alongside reusing and recycling, is vital for environmental restoration. However, at present, recycling costs exceed the production of new plastics from refined oil, hindering efforts to establish a circular plastic economy.
In conclusion, while plastics are flexible, durable, and remarkably versatile, our throwaway culture treats them as expendable. Chemistry reminds us that these materials were designed to endure, and it's our responsibility to manage their use and disposal wisely.