Innovative AI Tools Transforming Drug Discovery Processes
Written on
Chapter 1: Introduction to AI in Drug Discovery
The realm of drug discovery is evolving thanks to the integration of machine learning and AI technologies. These advancements are instrumental in various tasks, such as predicting protein folding, docking, and interactions. Such capabilities have significant implications in developing vaccines and addressing neurodegenerative diseases, among other applications in materials science. With the rise of machine learning and quantum computing, the biotech and medical fields are experiencing transformative changes.
One of the primary benefits of in-silico drug design lies in its ability to simulate countless variations, drastically reducing the costs and time associated with traditional trial-and-error methods. This optimization process is exemplified in the following video:
Section 1.1: Key Tools for Drug Discovery
One of the most renowned optimization tools is Ab-initio Rosetta, which utilizes the Monte Carlo method. However, the emergence of quantum annealers has begun to surpass these traditional methods by leveraging quantum phenomena such as tunneling. In addition, there are numerous machine learning-based applications worth noting. For further reading, consider this insightful article on the potential of quantum computing in drug development:
Quantum Landscape for Protein Folding
How quantum computing is holding the promise to develop drugs for neurodegenerative diseases and more
towardsdatascience.com
In this article, I will highlight some of the most notable open-source tools for drug discovery, including their GitHub repositories and related research papers:
- AlphaFold
- Generative Tensorial Reinforcement Learning (GENTRL)
- DeepChem
- Open Drug Discovery Toolkit (ODDT)
- Unified Rational Protein Engineering with Sequence-Based Deep Representation Learning (UNIREP)
Section 1.2: Spotlight on AlphaFold
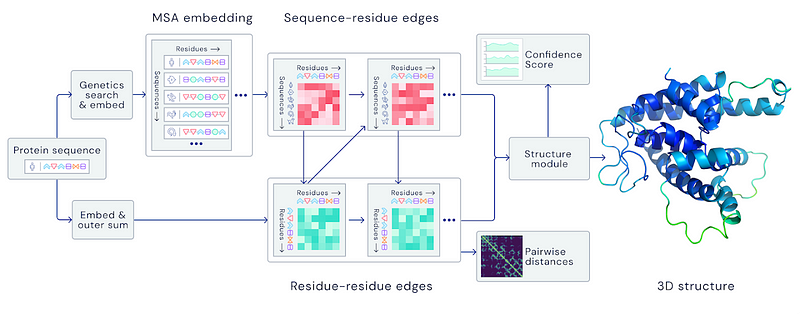
In recent years, AlphaFold from DeepMind has garnered significant attention in biotech news. The software versions released by DeepMind's team outperformed 97 other contenders in the biennial protein-structure prediction challenge known as the Critical Assessment of Structure Prediction (CASP) during its 13th and 14th editions. AlphaFold operates as a neural network that predicts the spatial structure of a protein based on its genetic code. Using input genetic sequences, it combines multiple sequence alignments, geometric representations, and deep learning techniques to derive the protein's structural graph.
The main distinction between AlphaFold versions lies in their neural network architectures: AlphaFold 1 employed Convolutional Neural Networks (CNNs), while AlphaFold 2 utilizes Transformers.
Chapter 2: Advanced Drug Discovery Techniques
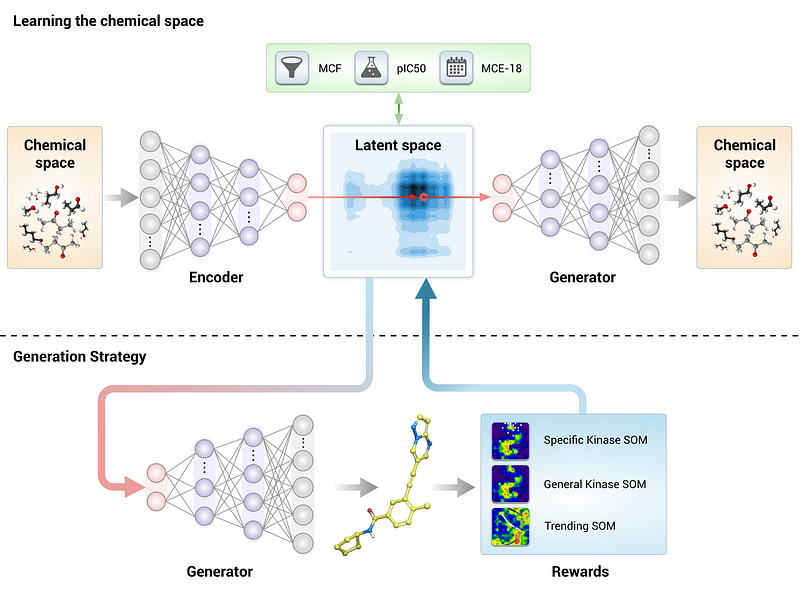
Generative Tensorial Reinforcement Learning (GENTRL) is another innovative tool developed by Insilico Medicine that accelerates experimental validation processes. Central to this tool is a variational autoencoder that employs tensor decompositions to capture the relationships between molecular structures and their properties, even accommodating datasets with missing values.
The GENTRL framework is based on reinforcement learning, following a streamlined pipeline for its applications.
DeepChem serves as an open-source deep learning platform designed to democratize drug discovery. Its capabilities include predicting the solubility of small drug-like molecules, estimating binding affinities for small molecules to protein targets, and analyzing protein structures to extract valuable descriptors. DeepChem employs Google TensorFlow and scikit-learn for machine learning tasks while utilizing RDKit for molecular data manipulations, such as converting SMILES strings into molecular graphs.
Open Drug Discovery Toolkit (ODDT) is a modular, comprehensive toolkit written in Python, aimed at cheminformatics and molecular modeling. Unlike other tools, ODDT does not rely on a popular deep learning library, requiring only scikit-learn. It incorporates advanced methods, including machine learning scoring functions like RF-Score and NNScore, allowing for customization in computer-aided drug discovery. For further details, reference the tutorial linked in the repository and the related paper published in the Journal of Cheminformatics.
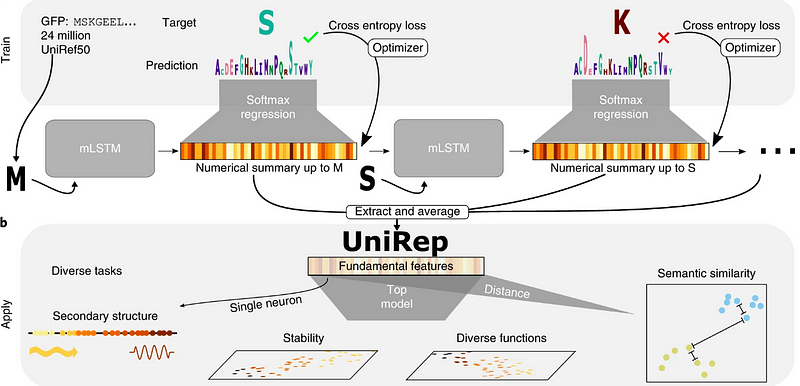
Unified Rational Protein Engineering with Sequence-Based Deep Representation Learning (UNIREP) employs a deep learning architecture that predicts protein features from unlabeled amino-acid sequences and phytochemical data. This method leverages Long Short-Term Memory (LSTM) networks to forecast the stability of both natural and designed proteins. The code is accessible on GitHub, and the corresponding reference paper was published in Nature Methods in 2019.
Concluding Thoughts
Despite the progress made, many pharmaceutical companies continue to face challenges in drug design due to substantial costs and lengthy timelines. Computational tools, particularly those powered by machine learning, present a promising avenue to streamline this process. For context, a standard high-throughput screening library may involve around one million compounds, with each compound costing between $50 to $100. Conducting a similar in-silico study requires only a capable computer, illustrating the potential for significant cost savings.
Nevertheless, it's crucial to recognize that deep learning models are often perceived as "black boxes." While considerable efforts have been made to integrate AI tools into the drug discovery lifecycle, further successful applications are necessary to fully realize AI's potential in this field. It is encouraging that even commercial entities are offering open-source tools and fostering community engagement to enhance their systems.
CONNECT WITH ME

@Dr_Alex_Crimi


